Nucleophilic asymmetric catalysis
We are interested in the development of chiral nucleophilic organocatalysts capable of promoting simple transformations of racemic (or achiral) substrates with high levels of asymmetric induction. In particular we are engaged in the development of chiral 4-dimethylaminopyridine analogues for use in a wide range of processes, including the kinetic resolution of racemates, the efficient asymmetric synthesis of molecules of biological and pharmaceutical interest and polymer synthesis. Early studies have led to the design of a chiral nucleophilic catalyst which can mimic enzymatic action by changing conformation on binding to its substrate (induced-fit model), which allows remote chiral information to influence the stereochemical course of the catalytic process. This design strategy allows chiral information to be transferred from catalyst to substrate in a controlled fashion that does not (as has often traditionally been the case) slow the reaction rate considerably.
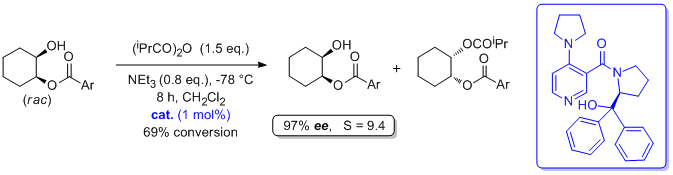
'(S)-Proline-derived catalysts for the acylative kinetic resolution of alcohols: a remote structural change allows a complete selectivity switch'
O. Gleeson, Y. K. Gun’ko and S. J. Connon*, Synlett 2013, 1728.
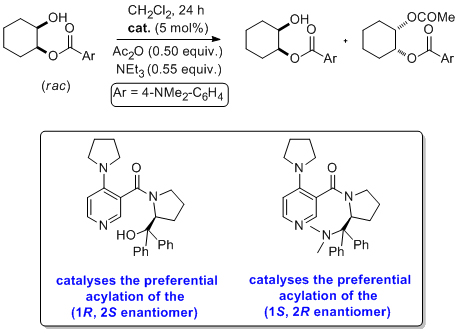
'Nonenzymatic acylative kinetic resolution of Baylis-Hillman adducts'
C. O. Dálaigh and S. J. Connon*, J. Org. Chem. 2007, 72, 7066.
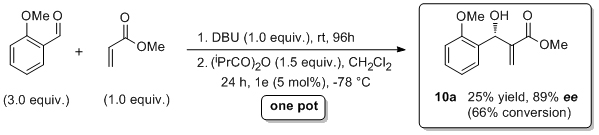
'Asymmetric acyl-transfer promoted by readily assembled chiral 4-N,N-dialkylaminopyridine derivatives'
C. O. Dálaigh, S. J. Hynes, J. E. O'Brien, T. McCabe, D. J. Maher, G. W. Watson and S. J. Connon*, Org. Biomol. Chem. 2006, 4, 2785.
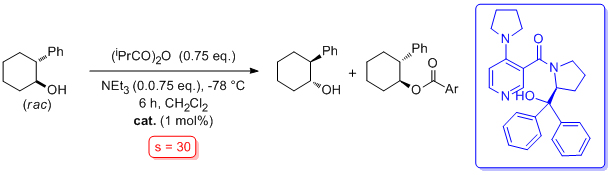
'Catalytic asymmetric acyl-transfer mediated by chiral pyridine derivatives'
S. J. Connon*, Lett. Org. Chem. 2006, 3, 333.
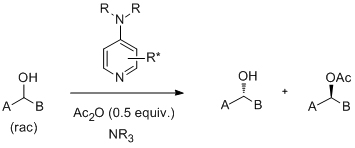
'Kinetic resolution of sec-alcohols using a new class of readily assembled (S)-proline-derived 4-(pyrrolidino)-pyridine analogues'
C. O. Dálaigh, S. J. Hynes, D. J. Maher and S. J. Connon*, Org. Biomol. Chem. 2005, 3, 981.